奧昔布寧
外观
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | Ditropan |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682141 |
| 核准狀況 | |
| 懷孕分級 |
|
| 给药途径 | 口服给药、transdermal gel、transdermal patch |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 血漿蛋白結合率 | 91–93% |
| 生物半衰期 | 12.4–13.2 hours |
| 识别信息 | |
| |
| CAS号 | 5633-20-5 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.590 |
| 化学信息 | |
| 化学式 | C22H31NO3 |
| 摩尔质量 | 357.486 g/mol |
| 3D模型(JSmol) | |
| |
| |
奧昔布寧(Oxybutynin),商品名Ditropan、Lyrinel XL、Lenditro (ZA)、Driptane (RU)、Uripan (Middle East)[1],為一種抗膽鹼劑,可以緩解膀胱排尿困難,包含頻尿及尿失禁等症狀,可以降低膀胱肌肉痙攣[2]。本品也可用於幫助緩解與腎結石相關的症狀。
本品可競爭性拮抗M1、M2,及M3等蕈毒鹼型乙醯膽鹼受體。
立體化學
[编辑]奧昔布寧含有一個立體中心,由兩種對映體組成。這是外消旋體,即1:(R)的1:1混合物 - 和(S)形式:[6][7]
| 奧昔布寧的對映體 | |
|---|---|
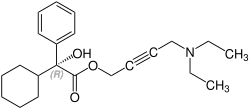 CAS-Nummer: 119618-21-2 |
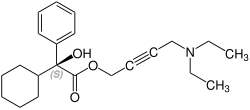 CAS-Nummer: 119618-22-3 |
參考文獻
[编辑]- ^ Uripan Tablets. Adwia Pharmaceuticals. [22 November 2015]. (原始内容存档于2020-10-26).
- ^ Chapple CR. "Muscarinic receptor antagonists in the treatment of overactive bladder". Urology (55)5, Supp. 1:33-46, 2000.
- ^ Tupker RA, Harmsze AM, Deneer VH. Oxybutynin therapy for generalized hyperhidrosis.. Arch Dermatol. 2006, 142 (8): 1065–6. PMID 16924061. doi:10.1001/archderm.142.8.1065.
- ^ Mijnhout GS, Kloosterman H, Simsek S, Strack van Schijndel RJ, Netelenbos JC. Oxybutynin: dry days for patients with hyperhidrosis.. Neth J Med. 2006, 64 (9): 326–8. PMID 17057269.
- ^ Schollhammer M, Misery L. Treatment of hyperhidrosis with oxybutynin.. Arch Dermatol. 2007, 143 (4): 544–5. PMID 17438194. doi:10.1001/archderm.143.4.544.
- ^ Kachur JF, et al. R and S enantiomers of oxybutynin: pharmacological effects in guinea pig bladder and intestine, Journal of Pharmacology and Experimental Therapeutics, 247, S. 867–872, 1988; PMID 2849672.
- ^ Noronha-Blob L, Kachur JF. Enantiomers of oxybutynin: in vitro pharmacological characterization at M1, M2 and M3 muscarinic receptors and in vivo effects on urinary bladder contraction, mydriasis and salivary secretion in guinea pigs, Journal of Pharmacology and Experimental Therapeutics, 256, S. 562–567, 1991; PMID 1993995.
