(E)-二苯基乙烯
| (E)-二苯基乙烯 | |
|---|---|

| |

| |
| IUPAC名 (E)-Stilbene[1] | |
| 英文名 | trans-stilbene |
| 识别 | |
| CAS号 | 103-30-0 |
| PubChem | 638088 |
| ChemSpider | 553649 |
| SMILES |
|
| InChI |
|
| InChIKey | PJANXHGTPQOBST-VAWYXSNFBV |
| ChEBI | 36007 |
| 性质 | |
| 化学式 | C14H12 |
| 摩尔质量 | 180.25 g·mol−1 |
| 外观 | 固体 |
| 密度 | 0.9707 g/cm3 |
| 熔点 | 122-125 °C |
| 沸点 | 305-307 °C |
| 溶解性(水) | 几乎不溶 |
| 危险性 | |
| MSDS | External MSDS |
| NFPA 704 | |
| 闪点 | >112 °C |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
(E)-二苯基乙烯是一种二芳基乙烯,这种烃类由反式的乙烯连接苯基基团所组成。与(Z)-二苯基乙烯互为顺反异构体。(E)-二苯基乙烯是白色晶体,极易溶于有机溶剂。它可以通过光化学的方法变成(Z)-二苯基乙烯,它可以继续被转化成菲。
(E)-二苯基乙烯于1843年由法国化学家奥古斯特·罗朗发现。[2]二苯基乙烯的英文名称“stilbene”来自希腊文 στίλβω (stilbo),意为闪耀,这来自于该化合物有光泽的外观。[3]
异构体
[编辑]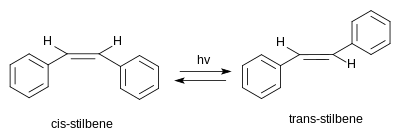
二苯基乙烯有两种可能的立体异构体。一种是反式-1,2-二苯基乙烯,又称(E)-二苯基乙烯;另一种是顺式-1,2-二苯基乙烯,又称(Z)-二苯基乙烯,它的位阻效应使得两个芳环不在一个平面上,共轭体系消失,更加不稳定。[4](Z)-二苯基乙烯是液体,熔点5—6 °C(41—43 °F),而(E)-二苯基乙烯是固体,熔点125 °C(257 °F),显示了它们明显不同的物理性质。[5][6]
制备
[编辑](E)-二苯基乙烯有多种制备方法。常见的方法包括使用锌汞齐还原苯偶姻:[6]
- C6H5–CH(OH)–C(=O)–C6H5 trans-C6H5–CH=CH–C6H5
二苯基乙烯的两种异构体都可以由α-苯基肉桂酸的脱羧反应而成。其中,(E)-二苯基乙烯是由这种酸的(Z)-异构体脱羧而成的。[5]
理查德·赫克[7]和沟吕木勉[8]独立报告了(E)-二苯基乙烯可以由碘苯和苯乙烯在钯(II)催化剂下偶联而成,也就是沟吕木-赫克反应。[9][10]沟吕木勉的方法产率较高。
反应
[编辑](E)-二苯基乙烯会被过氧磷酸(H3PO5)环氧化,在1,4-二氧六环中形成反式-二苯基环氧乙烷,产率74%。[11]这种环氧化物是外消旋混合物,由1,2-二苯基环氧乙烷的两种对映异构体组成。这种非手性的内消旋化合物 (1R,2S)-1,2-二苯基环氧乙烷来自(Z)-二苯基乙烯,尽管顺式异构体的环氧化会产生顺式和反式异构体的混合物。举个例子,过氧化叔丁醇氧化(Z)-二苯基乙烯会产生0.8% 顺式-二苯基环氧乙烷、13.5% 反式-二苯基环氧乙烷和 6.1% 苯甲醛。[12][13]诺贝尔奖得主巴里·夏普莱斯制备了纯二苯基环氧乙烷的一种对映异构体。[14]
二苯基乙烯可以被臭氧化反应[15]或勒米厄-约翰逊氧化反应干净的氧化成苯甲醛,而更强的氧化剂,如高锰酸钾的氧化会产生苯甲酸。邻位二醇可以通过厄普约翰双羟基化反应或夏普莱斯不对称双羟基化反应制备,[16][17]对映体过量百分数高达100%。[18][19][20]
溴化(E)-二苯基乙烯主要产生内消旋-1,2-二溴-1,2-二苯基乙烯,符合亲电溴加成反应会产生环状的溴钅翁离子中间体的机制。[21]
在紫外线照射下,(E)-二苯基乙烯会异构化成(Z)-二苯基乙烯。这是典型的光化学反应,涉及了顺反异构。它还可以继续转变成菲。[22]
衍生物和用处
[编辑](E)-二苯基乙烯本身的用处不多,但它的衍生物可用于染料、荧光增白剂、磷光体和闪烁体探测器。[23]二苯基乙烯是一种有源激光介质,用于染料激光器。[24]

4,4'-二硝基二苯基乙烯-2,2'-二磺酸钠可以由4-硝基甲苯的磺化先形成4-硝基甲苯-2-磺酸,然后被次氯酸钠氧化偶联成(E)-二苯基乙烯的衍生物。[25]这个过程最初是由Arthur George Green和André Wahl在19世纪开发的。[26][27]已经开发出更高产率的工艺改进,那就是在液氨中使用空气氧化。[28]这种物质很有用,和苯胺衍生物反应会得到偶氮染料。衍生于此化合物的重要染料包括Direct Red 76、Direct Brown 78和Direct Orange 40。[24]
参考资料
[编辑]- ^ International Union of Pure and Applied Chemistry. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. 2014: 379. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069.
- ^ Laurent, Auguste. Mémoire sur la série stilbique [Memoir on the stilbene series]. Comptes rendus. 1843, 16: 856–860 [2019-01-25]. (原始内容存档于2021-09-17) (法语). From p. 857: "En soumettant ce sulfure à la distillation, il donne plusieurs produits, et entre autres, un composé fort remarquable que je nomme stilbène." (On submitting this sulfide [i.e., phenyl thioaldehyde, C6H5(CS)H] to [dry] distillation, it gives several products, and among others, a very remarkable compound which I name "stilbene".)
- ^ Miller, William Allen. Elements of Chemistry: Theoretical and Practical 3 5th. London, England: Longmans, Green and Co. 1880: 366 [2019-01-25]. (原始内容存档于2021-08-01).
- ^ Eliel, Ernest L.; Wilen, Samuel H. Stereochemistry of Organic Compounds
 . John Wiley and Sons. 1994: 566-567. ISBN 0-471-01670-5.
. John Wiley and Sons. 1994: 566-567. ISBN 0-471-01670-5.
- ^ 5.0 5.1 (1953) "cis-Stilbene". Org. Synth. 33: 88; Coll. Vol. 4: 857.
- ^ 6.0 6.1 (1943) "trans-Stilbene". Org. Synth. 23: 86; Coll. Vol. 3: 786.
- ^ Heck, R. F.; Nolley, J. P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37 (14): 2320–2322. doi:10.1021/jo00979a024.
- ^ Mizoroki, Tsutomu; Mori, Kunio; Ozaki, Atsumu. Arylation of Olefin with Aryl Iodide Catalyzed by Palladium. Bull. Chem. Soc. Jpn. 1971, 44 (2): 581. doi:10.1246/bcsj.44.581
 .
.
- ^ Heck, Richard F. Palladium-catalyzed vinylation of organic halides. Org. React. 1982, 27: 345–390. ISBN 0471264180. doi:10.1002/0471264180.or027.02.
- ^ Beletskaya, Irina P.; Cheprakov, Andrei V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100 (8): 3009–3066. PMID 11749313. doi:10.1021/cr9903048.
- ^ Ogata, Yoshiro; Tomizawa, Kohtaro; Ikeda, Toshiyuki. Oxidation of trans-stilbene with peroxymonophosphoric acid. J. Org. Chem. 1979, 44 (14): 2362–2364. doi:10.1021/jo01328a006.
- ^ Yin, Guochuan; Danby, Andrew M.; Kitko, David; Carter, John D.; Scheper, William M.; Busch, Daryle H. Olefin Epoxidation by Alkyl Hydroperoxide with a Novel Cross-Bridged Cyclam Manganese Complex: Demonstration of Oxygenation by Two Distinct Reactive Intermediates. Inorg. Chem. 2007, 46 (6): 2173–2180. PMID 17295471. doi:10.1021/ic061957r.
- ^ Busch, Daryle H.; Yin, Guochuan; Less, Hyun-Jin. Lewis Acid Catalyzed Epoxidation of Olefins Using Hydrogen Peroxide: Growing Prominence and Expanding Range. Oyama, S. Ted (编). Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis. Elsevier. 2011: 119–153 [2021-08-01]. ISBN 9780080558011. (原始内容存档于2021-09-07).
- ^ Chang, Han-Ting; Sharpless, K. Barry. Molar Scale Synthesis of Enantiopure Stilbene Oxide. J. Org. Chem. 1996, 61 (18): 6456–6457. PMID 11667495. doi:10.1021/jo960718q.
- ^ Bishop, Clyde E.; Denson, Donald D.; Story, Paul R. Mechanisms of ozonolysis. The cis, trans-stilbene system. Tetrahedron Lett. 1968, 9 (55): 5739–5742. doi:10.1016/S0040-4039(00)76338-6.
- ^ Jacobsen, Eric N.; Marko, Istvan; Mungall, William S.; Schroeder, Georg; Sharpless, K. Barry. Asymmetric dihydroxylation via ligand-accelerated catalysis. J. Am. Chem. Soc. 1988, 110 (6): 1968–1970. doi:10.1021/ja00214a053.
- ^ Kolb, Hartmuth C.; VanNieuwenhze, Michael S.; Sharpless, K. Barry. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94 (8): 2483–2547. doi:10.1021/cr00032a009.
- ^ Wang, Zhi-Min; Sharpless, K. Barry. A Solid-to-Solid Asymmetric Dihydroxylation Procedure for Kilogram-Scale Preparation of Enantiopure Hydrobenzoin. J. Org. Chem. 1994, 59 (26): 8302–8303. doi:10.1021/jo00105a065.
- ^ (1992) "(R,R)-1,2-Diphenyl-1,2-ethanediol (Stilbene Diol)". Org. Synth. 70: 47; Coll. Vol. 9: 383.
- ^ Atta-ur-Rahman; Shah, Zahir. Asymmetric Hydroxylations. Stereoselectove Synthesis in Organic Chemistry. Springer-Verlag. 1993: 406–410 [2021-08-01]. ISBN 9781461383277. (原始内容存档于2021-08-01).
- ^ Gilbert, John C.; Martin, Stephen F. 10.6 – Bromination of Alkenes. Experimental Organic Chemistry: A Miniscale and Microscale Approach 5th. Cengage Learning. 2010: 376–383 [2021-08-01]. ISBN 9781439049143. (原始内容存档于2021-08-28).
- ^ Kwasniewski, S. P.; Claes, L.; François, J.-P.; Deleuze, M. S. High level theoretical study of the structure and rotational barriers of trans-stilbene. J. Chem. Phys. 2003, 118 (17): 7823–7836. Bibcode:2003JChPh.118.7823K. doi:10.1063/1.1563617.
- ^ Vogt, Peter F.; Gerulis, John J. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2000. ISBN 3527306730. doi:10.1002/14356007.a02_037.
|chapter=被忽略 (帮助) - ^ 24.0 24.1 Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. ISBN 3527306730. doi:10.1002/14356007.a03_245.
|chapter=被忽略 (帮助) - ^ Cumming, William M.; Hopper, I. Vance; Wheeler, T. Sherlock. Preparation 294.—Dinitro-Stilbene-Disulphonic Acid (Na salt). Systematic Organic Chemistry: Modern Methods of Preparation and Estimation. New York: D. Van Nostrand Company. 1926: 314.
- ^ Green, Arthur G.; Wahl, André R. Ueber die Oxydation von Paranitrotoluolsulfosäure [On the oxidation of para-nitrotoluenesulfonic acid]. Ber. Dtsch. Chem. Ges. 1897, 30 (3): 3097–3101 [2021-08-01]. doi:10.1002/cber.189703003128. (原始内容存档于2021-11-28) (德语).
- ^ Green, Arthur G.; Wahl, André R. Ueber die Oxydation der Paranitrotoluolsulfosäure [On the oxidation of para-nitrotoluenesulfonic acid]. Ber. Dtsch. Chem. Ges. 1898, 31 (1): 1078–1080 [2021-08-01]. doi:10.1002/cber.189803101195. (原始内容存档于2021-11-28) (德语).
- ^ US patent 5041632,Guglielmetti, Leonardo,「Process for the preparation of 4,4'-dinitrostilbene-2,2-disulfonic acid」,发表于1991-08-20,发行于1991-08-20,指定于Ciba-Geigy Corporation


![{\displaystyle {\ce {->[{\ce {Zn(Hg)}}][{\ce {HCl}}{\text{, }}{\ce {CH3CH2OH}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e1406e12754001b43f053ffef11d727e4d7de0e9)


