嗎啡全合成
外觀

嗎啡全合成是指化學中嗎啡樣生物鹼的合成,描述了天然嗎啡喃類生物鹼的全合成,其包括可待因,嗎啡,奧利平,和蒂巴因及其密切相關的半合成類似物丁丙諾啡,氫可酮,異可待因,納曲酮,納洛酮,納布啡和羥考酮[1][2]。
嗎啡的結構並不是特別複雜,然而,相鄰結合原子的靜電極化在整個結構中不均勻交替。這種「不一致的連接性」使得鍵的形成更加困難,並且因此使應用於該分子家族的任何合成策略明顯的複雜化[2]。
首次全合成由美國化學家馬歇爾·D·蓋茨於1952年完成,並被視為這個領域中的經典之作[3]。該合成總共用了31個步驟,並且總產率有0.06%。Kenner C. Rice的氫可酮合成是最有效的,用了14個步驟並且有30%的總產率[4]。
此後,許多化學家提出了新的合成路線,其中值得注意的有以下研究者率領的團隊所提出的路線:賴斯、[5]埃文斯、[6]富克斯、[7]帕克、[8]奧爾曼、[9]木澤爾·特勞納、[10]懷特、[11]泰伯、[12]特羅斯特、[13]福山、[14]吉尤[15]和斯托克。[16]
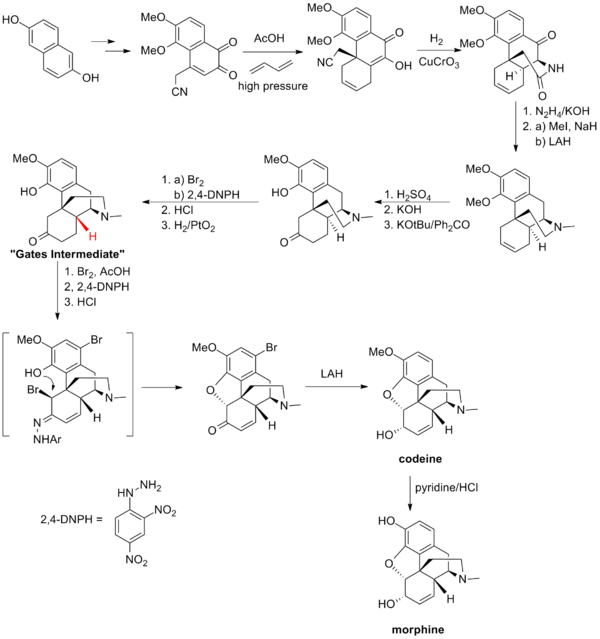
蓋茨的合成路線
[編輯]蓋茨的嗎啡全合成路線是在全合成中運用Diels-Alder反應的首個例子。
Rice的合成路線
[編輯]Rice合成遵循仿生途徑,並且是迄今為止已經被報道中最有效的。一個關鍵的步驟是Grewe環化,類似於發生在嗎啡生物合成中的網狀結構的環化 [4]。
參考文獻
[編輯]- ^ Chida N. Recent advances in the synthesis of morphine and related alkaloids. Top Curr Chem. 2011, 299: 1–28. PMID 21630507. doi:10.1007/128_2010_73.
- ^ 2.0 2.1 Rinner U, Hudlicky T. Synthesis of morphine alkaloids and derivatives. Top Curr Chem. 2012, 309: 33–66. PMID 21547687. doi:10.1007/128_2011_133.
Morphine's synthesis remains a serious challenge to this day.
- ^ Gates, Marshall; Tschudi, Gilg. Journal of the American Chemical Society. 1956, 78 (7): 1380. doi:10.1021/ja01588a033. 缺少或
|title=為空 (幫助) - ^ 4.0 4.1 Rice KC. Synthetic opium alkaloids and derivatives. A short total synthesis of (+/-)-dihydrothebainone, (+/-)-dihydrocodeinone, and (+/-)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners. The Journal of Organic Chemistry. July 1980, 45 (15): 3135–3137. doi:10.1021/jo01303a045.
- ^ Rice, Kenner C. Synthetic opium alkaloids and derivatives. A short total synthesis of (.+-.)-dihydrothebainone, (.+-.)-dihydrocodeinone, and (.+-.)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners. The Journal of Organic Chemistry. 1980, 45 (15): 3135. doi:10.1021/jo01303a045.
- ^ Evans, D.A.; Mitch, C.H. Studies directed towards the total synthesis of morphine alkaloids. Tetrahedron Letters. 1982, 23 (3): 285. doi:10.1016/S0040-4039(00)86810-0.
- ^ Toth, J. E.; Hamann, P. R.; Fuchs, P. L. Studies culminating in the total synthesis of (dl)-morphine. The Journal of Organic Chemistry. 1988, 53 (20): 4694. doi:10.1021/jo00255a008.
- ^ Parker, Kathlyn A.; Fokas, Demosthenes. Convergent synthesis of (.+-.)-dihydroisocodeine in 11 steps by the tandem radical cyclization strategy. A formal total synthesis of (.+-.)-morphine. Journal of the American Chemical Society. 1992, 114 (24): 9688. doi:10.1021/ja00050a075.
- ^ Hong, Chang Y.; Kado, Noriyuki; Overman, Larry E. Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (-)- and (+)-dihydrocodeinone and (-)- and (+)-morphine. Journal of the American Chemical Society. 1993, 115 (23): 11028. doi:10.1021/ja00076a086.
- ^ Mulzer, Johann; Dürner, Gerd; Trauner, Dirk. Formal Total Synthesis of(—)-Morphine by Cuprate Conjugate Addition. Angewandte Chemie International Edition in English. 1996, 35 (2324): 2830. doi:10.1002/anie.199628301.
- ^ White, James D.; Hrnciar, Peter; Stappenbeck, Frank. Asymmetric Total Synthesis of (+)-Codeine via Intramolecular Carbenoid Insertion. The Journal of Organic Chemistry. 1999, 64 (21): 7871. doi:10.1021/jo990905z.
- ^ Taber, Douglass F.; Neubert, Timothy D.; Rheingold, Arnold L. Synthesis of (−)-Morphine. Journal of the American Chemical Society. 2002, 124 (42): 12416–7. PMID 12381175. doi:10.1021/ja027882h.
- ^ Trost, Barry M.; Tang, Weiping. Enantioselective Synthesis of (−)-Codeine and (−)-Morphine. Journal of the American Chemical Society. 2002, 124 (49): 14542–3. PMID 12465957. doi:10.1021/ja0283394.
- ^ Uchida, Kenji; Yokoshima, Satoshi; Kan, Toshiyuki; Fukuyama, Tohru. Total Synthesis of (±)-Morphine. Organic Letters. 2006, 8 (23): 5311–3. PMID 17078705. doi:10.1021/ol062112m.
- ^ Varin, Marie; Barré, Elvina; Iorga, Bogdan; Guillou, Catherine. Diastereoselective Total Synthesis of (±)-Codeine. Chemistry - A European Journal. 2008, 14 (22): 6606. doi:10.1002/chem.200800744.
- ^ Stork, Gilbert; Yamashita, Ayako; Adams, Julian; Schulte, Gary R.; Chesworth, Richard; Miyazaki, Yoji; Farmer, Jay J. Regiospecific and Stereoselective Syntheses of (±) Morphine, Codeine, and Thebaine via a Highly Stereocontrolled Intramolecular 4 + 2 Cycloaddition Leading to a Phenanthrofuran System. Journal of the American Chemical Society. 2009, 131 (32): 11402–6. PMID 19624126. doi:10.1021/ja9038505.